EXCESS SALINATION
Humic acids can reduce the over-salination problem in the soil, especially in regions where too much irrigation has resulted in toxicities. In soils with excess salination, humic acids can fix anions and cations and eliminate them from the roots region of plants and provide a compensation for the deficit of organic substances.
One of the best examples for excess salination has been observed in Egypt with the construction of the Assuan dam in 1970, which intervened in the natural flow of the Nile river. One of the consequences was the elimination of the load (ton-humus complexes) of the river for soils as a natural fertilizer , which supplied for nutrients and humic acids. Another effect was the salination of soils through equally high level of groundwater. As a result, Egypt became worldwide one of the biggest importers of fertilizers in only about 10 years. The reduced flow speed of the Nile led to an accumulation of the nutrients essential for fertile soils in the dam lake and consequently to a huge deficits of nutrients in the arable land.
 image of Highly salinized soil
image of Highly salinized soil
pic.1: Highly salinized soil.
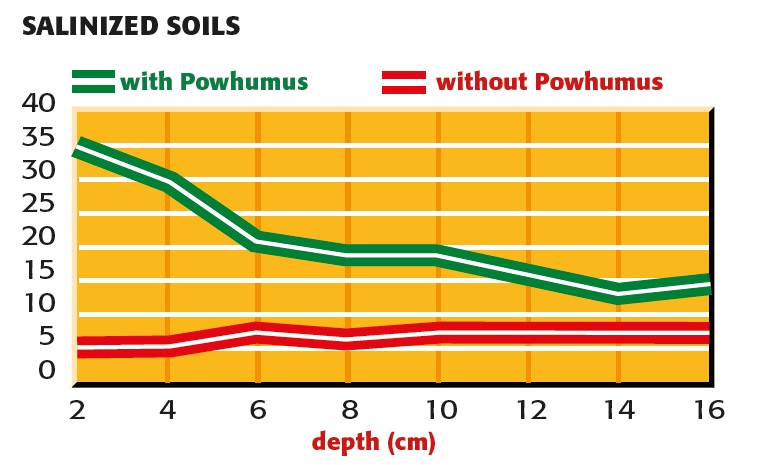 Splitting of salt with and without powhumus
Splitting of salt with and without powhumus
fig.1: Splitting of salt
The steady changes in the level of groundwater before the construction of the dam had enabled a continuous erosion of the soil, which guaranteed that there was not a concentration of undesired anions and cations in the upper layers. With a continuous high level of groundwater following the construction of the dam, this kind of concentration occured indeed. The consequent salination and alkalifying of soils leads naturally to losses in crop yields and even to a complete abandonment of arable land. This type of radical intervention in the ecological system has had similar or same consequences in other countries as well. The ions responsible for salination are:
This type of radical intervention in the ecological system has had similar or same consequences in other countries as well. The ions responsible for salination are:
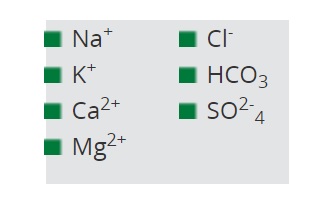 chemicals for which humic acids have fixation properties
chemicals for which humic acids have fixation properties
Research has shown that humic acids have fixation properties for the following:
- Na+ and Cl- ions are tied up by humic acids.
- NaHCO3 is adsorbed after the L-type isotherm.
- The fixation of Mg2+ and Ca2+ by humic acids has been known for a long time.
On the one hand, these salts are adsorbed by the surface of the humic acids, and on the other hand, their ions can also be fixed electrostically by the corresponding functional groups (carboxyl and OH-groups).
The property of humic acids of forming complexes with polyvalent cations is of great relevance here. Salts with bivalent ions are fixed more strongly as expected. More significantly, this type of fixation (adsorption) takes place relatively with little binding energy, as a result of which a disruption in the balance takes place with the sinking of ground water during dry phases and thus results in a release of ions and their bonds to the deeper layers of the earth back to their original place.
1. Fixation of metal ions (Me+) :
2. Fixation of anions: humic acids as bases:
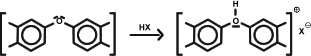 Fixation of anions: humic acids as bases
Fixation of anions: humic acids as bases
An experimental proved reduction in salination of soils in Egypt through humic acids:
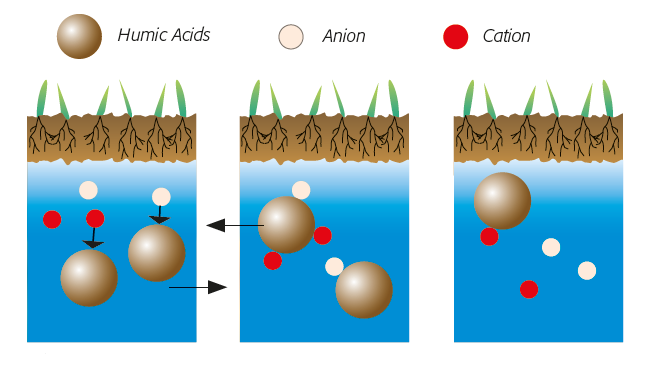
I. Earlier state of the soils before the construction of the Assuan Dam
Release of salts from the deeper layers of the soil, with sinking ground water: taken back to the deeper layers of the soil.
II.1 State of soils after the construction of the Assuan Dam without humic acids
Out-crystallization of salts in the top horizont with permanent accumulation: salination
II.2 State of soils after the construction of the Assuan Dam in the presence of humic acids
Fixation of ions (1) and solubility balance (2). Little mobile humic acids act as a barrier in the upper layers: no salination (3).
Some of the information on this page has been derived from Wolfgang Ziechmann, Huminstoffe und ihre Wirkungen, Spektrum Akademischer Verlag, Heildelberg - Berlin - Oxford, 1996.
 depiction of how Humic acids reduce the effects of salinity
depiction of how Humic acids reduce the effects of salinity
fig.2: Humic acids reduce the effeczs of salinity.
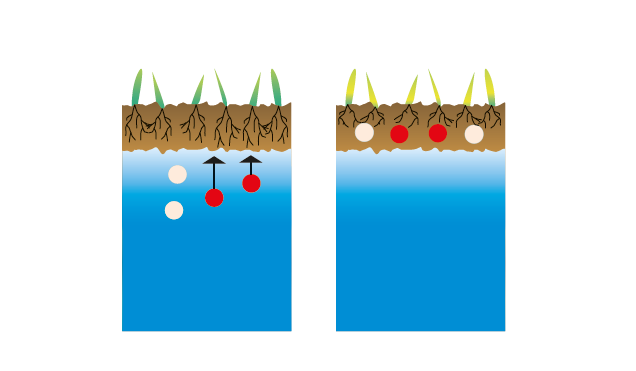
fig.3: Salinized groundwater in a soil.


