Am Pösenberg 9-13
D-41517 Grevenbroich
Telefon: +49 2181 70 676 - 0
Fax: +49 2181 70 676 - 22
E-Mail senden
What are Humic Acids?
1. What are humic acids benefits at environmental applications?
Humic matteris formed through the chemical and biological humification of plant and animal matter and through the biological activities of micro-organisms. The biological center, the main fraction of natural humic matter, are the humic acids, which contain humic acid and fulvic acid. Humic acids are an excellent natural and organic way to provide plants and soil with a concentrated dose of essential nutrients, vitamins and trace elements. They are complex molecules that exist naturally in soils, peats, oceans and fresh waters. The best source of humic acids are the sedimentation layers of soft brown coal, which are referred to as Leonardite. Humic acids are found in high concentration here. Leonardite is organic matter, which has not reached the state of coal and differs from soft brown coal by its high oxidation degree, a result of the process of coal formation (bog>peat>coal), and high humic acids content as well as higher carboxyl groups.
Compared to other organic products, oxidized lignite is very rich in humic acids. While oxidized lignite is the end product of a humification process of several million years, the formation period of peat, for instance, is completed within only a few thousand years.
2. Benefits of humic acids at environmental applications
Humic substances in general and humic acids in detail interact with all classes of ecotoxicants including: heavy metals, petroleum and chlorinated hydrocarbons, pesticides, nitroaromatic explosives, azo dyes, actinides, etc.
Humic acids are known to form stable complexes with heavy metals and adducts with hydrophobic organic compounds; produce charge-transfer complexes; act as electron shuttles and mediate redox reactions of transition metals, of chlorinated and nitrated hydrocarbons; adsorb onto mineral surfaces; and influence the interphase distribution of the contaminants. Finally, humics can strengthen the resistance of living organisms against non-specific stress factors.
This unique constellation of reactive features strongly suggests humic substances have the potential to address a broad spectrum of needs within the focus area of environmental remediation. This theoretical statement is confirmed by multiple examples of actual applications in remediation. However, to ensure optimum and systematic application, an expanded knowledge base is needed concerning interactions between humics, ecotoxicants, and living organisms.
Multiple interactions between humic substances / humic acids, ecotoxicants, and living organisms may be organized to include:
- binding interactions that impact chemical speciation and bioavailability of ecotoxicants;
- sorptive interactions affecting physical speciation or interphase partitioning of ecotoxicants;
- abiotic-biotic redox interactions that impact metabolic pathways coupled toecotoxicants; and,
- direct and indirect interactions with various physiological functions of living organisms.
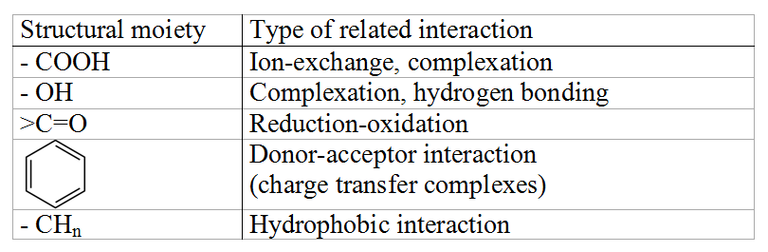
Fig.1: Humics can take part in protolytic, ion exchange, and complexation reactions; participate in donor-acceptor interactions; engage in hydrogen bonding; and take part in van-der-Waals interactions.
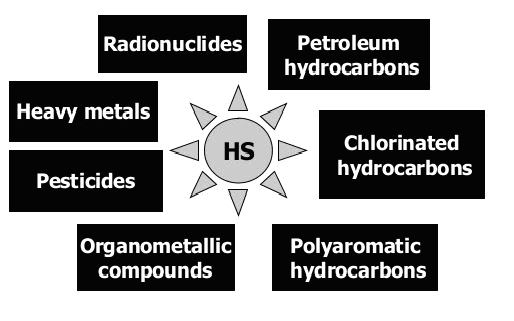
Fig. 2: As a result of the diverse reactivity of HS, they can interact with all classes of ecotoxicants (ET) in the polluted environment. Humics are known to form stable complexes with heavy metals and radionuclides; to produce adducts and charge transfer complexes with hydrophobic organic compounds; to mediate redox reactions of transition metals, chlorinated and nitrated hydrocarbons.
(from: I.V. PERMINOVA, K. HATFIELD, REMEDIATION CHEMISTRY OF HUMIC SUBSTANCES: THEORY AND IMPLICATIONS FOR TECHNOLOGY)

